Oxaliplatin-associated sarcoid-like reaction masquerading as recurrent colon cancer
- 1 Internal Medicine, Carle Foundation Hospital, Urbana, Illinois, USA
- 2 Internal Medicine, University of Illinois College of Medicine at Peoria, Peoria, Illinois, USA
- 3 Medical Oncology, University of Kansas Medical Center, Kansas City, Kansas, USA
- Correspondence to Dr Anup Kasi; anupdoc@gmail.com
Abstract
A 54-year-old man with stage IV B metastatic colorectal cancer with liver and peritoneal metastasis was treated with cytoreductive surgery (extended left colectomy, right partial hepatectomy, resection of right diaphragm nodule) and perioperative oxaliplatin-based chemotherapy. The patient was cancer-free for 6 months, at which point a surveillance positron emission tomography-CT scan showed metabolically active hepatosplenic lesions and mediastinal and bilateral hilar lymph nodes. An endobronchial ultrasound bronchoscopy-guided fine needle aspiration of the mediastinal and hilar lymph nodes revealed non-necrotising granulomas. The workup was negative for bacterial, fungal or mycobacterial infection, cancer or autoimmune disease. Carcinoembryonic antigen and COLVERA (a circulating tumour DNA liquid biopsy test for the detection of recurrent colon cancer) tests were negative. Subsequently the rare diagnosis of a sarcoidosis-like reaction from oxaliplatin-based chemotherapy was made. Repeat imaging after 3 months showed resolution of the hepatosplenic lesions and lymphadenopathy, alike.
Background
The standard, first-line treatment for metastatic colorectal cancer is a combination of oxaliplatin or irinotecan,1–3 short-term infusion fluorouracil and leucovorin. The common side effects of this treatment regimen are haematological (neutropenia), gastrointestinal and neurological (acute neurosensory complex, dysesthesias).4 Acute lung injury sometimes occurs with oxaliplatin chemotherapy, but granulomatous disorders are rarely seen. We are reporting a rare case of a sarcoid-like reaction with oxaliplatin-based chemotherapy.
Case presentation
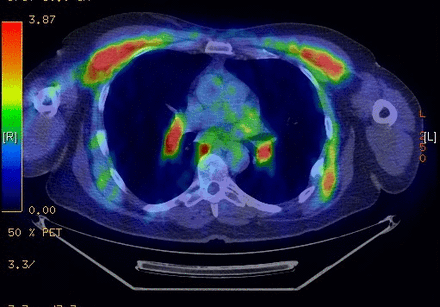
A 54-year-old Caucasian man with no history of smoking visited our hospital for surveillance of colon cancer. He had been diagnosed with stage IV B metastatic colon adenocarcinoma 12 months earlier. He presented with obstructive bowel symptoms, a change in stool calibre, and weight loss of 40 pounds over 6 months. The initial positron emission tomography (PET)-CT scan showed focal colonic bowel wall thickening which raised concern for a mass involving the distal transverse colon, as well as hypodensities in the right lobe of the liver, which raised concern for metastatic disease. A colonoscopy showed a mass located in the transverse colon and subsequent biopsy revealed a poorly differentiated mucinous adenocarcinoma that was ulcerated and invasive with accompanied desmoplasia. Due to his severe obstructive bowel symptoms, the patient was taken to the operating room. He underwent an extended left colectomy, right partial hepatectomy, resection of the right diaphragmatic nodule and cholecystectomy. Standard-of-care, next-generation sequencing was performed by a clinical laboratory improvement amendment-approved lab which revealed a Kirsten rat sarcoma (KRAS)5 mutation. The patient then received systemic chemotherapy with a combination of folinic acid/5-fluorouracil/oxaliplatin+bevacizumab for six cycles, which led to an excellent partial-response. Next he had cytoreductive surgery as well as an exploratory laparotomy and omentectomy, followed by six additional cycles of chemotherapy. His post-treatment imaging, carcinoembryonic antigen (CEA) and a circulating tumour DNA liquid biopsy test for the detection of recurrent colon cancer (COLVERA) were negative for recurrence. Six months following his completion of chemotherapy, a surveillance PET-CT scan showed mediastinal and bilateral hilar lymphadenopathy along with scattered hepatic and splenic lesions, which were observed to be metabolically active on a subsequent PET scan (figure 1). At that time, the patient was afebrile and denied weight loss, change in appetite or any respiratory symptoms. Physical examination did not show any extrapulmonary lesions. While there was initially concern for recurrent cancer, the CEA and COLVERA blood tests were negative, making that possibility unlikely. A subsequent endobronchial ultrasound bronchoscopy (EBUS) of the right paratracheal and interlobar lymph nodes was obtained that revealed non-caseating granulomas without evidence of malignant cells (figure 2). The patient had lived all his life in Kansas, did not have a significant history of travel outside USA, exposure to animals and no known exposure to tuberculosis. The patient’s ACE level was elevated at 69 µL (normal: 8–53 µL), and his serum calcium level was elevated at 10.7 mg/dL. As he was asymptomatic, he was observed without any further interventions. Three months later, a repeat CT scan demonstrated resolution of the lymphadenopathy and hepatosplenic lesions. His serum ACE and calcium levels had also normalised in the interim. Thus, the rare diagnosis of a sarcoidosis-like reaction from oxaliplatin-based chemotherapy was made. We have now followed the patient with surveillance CT scans every 3 months for 18 months with no evidence of recurrent lymphadenopathy or hepatosplenic lesions or cancer.
Positron emission tomography scan showing hypermetabolic mediastinal and bilateral hilar lymphadenopathy.
H&E, cell block preparation from endobronchial ultrasound-guided fine needle aspiration specimen of the right paratracheal lymph node. Sections show a well-formed non-necrotising granuloma composed of an aggregate of histiocytes with associated lymphocytes.

Investigations
As described earlier, a surveillance PET-CT scan performed on the patient with a history of metastatic colorectal cancer showed mediastinal and hilar lymph nodes and scattered hepatic and splenic lesions. These were demonstrated to be hypermetabolic on PET scan (figure 1). An EBUS biopsy of the right paratracheal and interlobar lymph nodes revealed non-caseating granulomas. Post-treatment CEA and COLVERA where negative for cancer recurrence. Bronchoalveolar lavage (BAL) fluid cultures were negative for fungal and aerobic bacterial cultures and mycobacterium tuberculosis. Acid-fast bacilli and Grocott’s methenamine silver (GMS) fungal stains of hilar lymph node specimens were negative for acid-fast bacteria and fungal organisms. Other tests (histoplasma antigen), T-SPOT (a blood test for TB), serum cryptococcal antigen, bartonella panel and brucellosis antibodies were negative. Fungitell assay and galactomannan assay tests were also negative. Autoantibodies screening for antinuclear antibodies, rheumatoid factor and antineutrophil cytoplasmic antibodies was negative as well. The patient’s ACE level was found to be elevated at 69 µL (normal: 8–53 µL), and his serum calcium level was found to be elevated at 10.7 mg/dL.
Differential diagnosis
The presence of enlarged lymph nodes as well as hepatic and splenic lesions in the setting of malignancy first indicated recurrence of cancer or metastasis. Non-caseating granulomas then pointed towards an infectious aetiology, such as atypical tuberculosis, brucellosis or various fungal infections. Other differential diagnosis such as connective tissue disorders namely Wegner’s granulomatosis were ruled out in our case. Granulomatous and lymphocytic interstitial lung disease are also a possibility, this is usually associated with common variable immunodeficiency and immunodeficiency state, but typically this condition does not resolve spontaneously.
Outcome and follow-up
Repeat imaging after 3 months showed resolution of the hepatosplenic lesions and lymphadenopathy. Repeat CEA tumour marker and COLVERA tests were also negative. The patient remains cancer-free and continues to be monitored.
Discussion
Commonly seen causes of mediastinal and hilar lymph node enlargement in patients with a history of cancer who have been treated with chemotherapy include recurrent cancer, infection, unrelated primary malignancy, drug side effects and radiative lung injury. Infections are the most common causes, which were ruled-out in this case via extensive workup of the biopsy specimen and BAL fluid analysis.
Investigation of the biopsy specimen revealed the presence of non-caseating granulomas, which ruled out neoplasia.
The platinum-derivative chemotherapeutic agent oxaliplatin was approved by the Food and Drug Administration (FDA) for treatment of metastatic colorectal cancer in 2002. Since then it has been widely used.6 Oxaliplatin inhibits DNA synthesis by causing inter-strand and intra-strand crosslinks. The most common toxicities of oxaliplatin-based chemotherapy are haematological (13%–52%), gastrointestinal (10%–33%) and neurological complications (85%).4 Pulmonary toxicities, while rare, have been reported and include diffuse alveolar damage, non-specific interstitial pneumonia, eosinophilic pneumonia, interstitial lung disease and organising pneumonia.5 7–12 The time from initial exposure to lung injury has varied from 1 day to more than 6 months. Our case illustrates the development of a sarcoidosis-like reaction that occurred 6 months after treatment with an oxaliplatin-based chemotherapy. Only one such case has been reported so far; however, there may be more that have not yet been diagnosed or reported because biopsies are not always taken.13
A drug-induced sarcoid-like reaction (DISR) is indistinguishable from sarcoidosis. It occurs after exposure to an offending agent.14 DISR presents similar to sarcoidosis as a bilateral hilar adenopathy, with associated cutaneous lesions, hypercalcemia and elevated levels of serum ACE. The common classes of drugs associated with the development of such a sarcoid-like reaction are anti-neoplastic drugs, such as everolimus, gefitinib, antiretroviral drugs, interferons,15 methotrexate and tumour necrosis factor-alpha antagonists.16 To our knowledge, sarcoidosis-like reaction from oxaliplatin-based chemotherapy has been reported in a single case report previously.13
Diagnosis of sarcoidosis can be made based on a combination of clinical features, supporting laboratory findings, such as elevated calcium levels and ACE levels, radiological findings and a biopsy demonstrating non-caseating granulomas. In addition, other granulomatous diseases must be excluded. Our patient’s biopsy was consistent with non-caseating granulomas, his ACE and calcium levels were elevated, and other alternative diagnosis, such as recurrence of cancer and causes of non-caseating granulomatous diseases were excluded, leading to the diagnosis of sarcoidosis.
DISR usually resolves after withdrawal of the offending agent. For this reason, clinicians should carefully consider the benefit of continuing the drug versus the severity of the toxicity. If the benefit from continuing the drug outweighs the effects of DISR, the drug could be continued.15 Similar to patients with sarcoidosis, patients with DISR do not require treatment unless their symptoms are significantly bothersome, the sarcoidosis progresses in a way it begins to affect their quality-of-life, or end-organ dysfunction occurs. Corticosteroids and other drugs such as methotrexate and azathioprine can be used in patients with progressing or severe symptoms. In some patients, switching to a different drug within the same class as the offending agent may result in resolution of the symptoms.16 Our patient was asymptomatic, so no treatment was given, but he was monitored closely with frequent imaging. Repeat serum ACE and calcium levels after 3 months returned to within normal limits. CT scans after 3 months demonstrated resolution of the hepatosplenic lesions and lymphadenopathy.
In conclusion, although the exact mechanism of this injury should be investigated further, oxaliplatin-based chemotherapy should be considered a possible cause of drug-induced sarcoidosis, and withdrawal may be sufficient for control of subsequent adverse events. If symptoms persist or progress and the use of the offending agent is determined to be more beneficial than the presenting symptoms, traditional sarcoidosis treatment options including steroids may be used. When patients treated with oxaliplatin-based regimens develop otherwise unexplained lymphadenopathy with lesions in various organs including the liver and spleen, drug-induced sarcoidosis should be included in the differential diagnosis, as it was in this case.
Patient’s perspective
The day I was first told I have cancer will stay in my mind forever. I was on the phone with a friend and could tell my wife of 32 years had something bothering her. I asked her what was wrong and she said ‘nothing’ several times. She finally told me I had cancer. She said the doctor didn't want to tell me while still groggy from the anaesthesia and she had the choice to tell me or wait until the following week at my follow-up appointment and the doctor would advise me. She knew she could not keep it from me that long.
At first it was like a brick wall hitting me, I am a religious man and always had the opinion that if it is your time to go, it is your time and only God knows what will happen. I am a grandpa to three wonderful grandchildren and two great daughters that I need to take care of and be there for.
My family told me it was my decision whether I wanted to do nothing or go through treatment. They would stand behind my decision. I decided I wanted to be around for my grandchildren, daughters and the love of my life so I chose treatment. I was scared, but again I knew the Lord had me in his hands and would take care of me. My first surgery went very well, although they found the cancer had went through the colon wall into my peritoneum area. The doctors removed two plum size masses and 12 inches of my colon as well as several small portions of my liver. An access port was surgically implanted to prepare for chemo which began about a month after my first surgery.
I did not know what to expect but knew my Lord and my family were with me and supported my decision. I went through six rounds of chemotherapy. I was very fortunate as I had no real major side effects. I was not sick to my stomach and did not loose my hair, mostly just tired. Because the cancer had spread outside the colon, I later had the cryoreductive surgery and heated intraperitoneal chemotherapy (CRS HIPEC) surgery. The doctors felt they had removed all the liver cancer cells but wanted to follow-up with six more rounds of chemo to be certain.
I have been cancer-free for a little over a year. This was very aggressive treatment, but the doctors choice of surgeries and chemo worked miracles for me. The support of family and friends also helped me through the treatment. Keeping a positive attitude is a MUST.
I owe my life to the doctors and nurses at KU Med Center. I am slowly getting back to normal after 2 years. It definitely changes your perspective on life, you quit letting the small things bother you and appreciate what you have. I appreciate the opportunity to share my experience.
Learning points
-
If recurrent colon cancer is suspected based on imaging and a carcinoembryonic antigen test is normal, then a negative circulating tumour DNA-based test such as COLVERA (circulating tumour DNA liquid biopsy test for the detection of recurrent colon cancer) test may aid to rule out colon cancer recurrence.
-
After ruling out colon cancer recurrence, suspect a drug-induced sarcoidosis-like reaction when you encounter a case of thoracic lymphadenopathy with or without hepatosplenic lesions occurring after treatment with oxaliplatin-based chemotherapy.
-
Discontinuation of an offending drug may be sufficient to control, such a sarcoidosis-like reaction. If symptoms persist or progress, and the use of the offending drug is determined to more beneficial, treatment options including steroids and other agents used for the treatment of sarcoidosis could be considered.
Footnotes
-
Contributors SKA was involved in reviewing the patient medical records, acquisition of data, reviewing previously published literature and drafting the manuscript. AC and SKA coordinated with other authors to streamline the content to make it clinically and scientifically meaningful. SK provided images of biopsy and figure legends. She also helped with the interpretation of data and edited the content. AK was involved in the conception and design of the case report. He revised it critically for important intellectual content before providing the final approval. He obtained patient consent. He agrees to be accountable for the article and to ensure that all questions regarding the accuracy or integrity of the article are investigated and resolved.
-
Funding The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
-
Competing interests None declared.
-
Patient consent for publication Obtained.
-
Provenance and peer review Not commissioned; externally peer reviewed.
- © BMJ Publishing Group Limited 2020. No commercial re-use. See rights and permissions. Published by BMJ.
References
Use of this content is subject to our disclaimer